
– By Tanya Agarwal (Penultimate Year Student at Amity Law School Delhi, GGSIP University)
– By Tanya Patwal (Penultimate Year Student at Amity Law School Delhi, GGSIP University)
ABSTRACT
When India declared its initiative of delivering paracetamols and hydroxychloroquine and later gift the two made in India vaccines to multiple countries throughout the world, a new era of diplomacy was ushered. India’s “vaccine diplomacy” was seen as an unprecedented display of humanity and the concept of “love thy neighbor”. India’s further actions at the WTO, where it sought for complete waiver of patents on COVID-19 so that lesser developed nations do not fall behind in the queue for vaccination further cemented this image.
However, India’s arguments at the WTO, while seen as a stand for the right cause, is rather myopic. Not only do they seek to suppress the rights of those who worked hard to develop groundbreaking vaccines at the height of a pandemic, they put at great risk the future of pharmaceutical research. India’s advocacy for easier and inexpensive access to vaccines may seem noble, however it fails to understand that patenting the vaccines will not necessarily hamper the vaccination drive in countries that cannot afford it, with a little help from other nations.
In this paper, the authors set out to explain why patenting of vaccines is important and how it can be done without compromising on global healthcare and India’s commitment towards humanitarian aid. They first lay down in brief the nexus between trade and patents, India’s status on patenting COVID-19 vaccines and then establish why patents are important and how India should go about it without giving up on its unfortunate global peers
INTRODUCTION
Amidst a global pandemic, India volunteered to help the world when it first supplied medicines and then gifted and distributed millions of doses of two vaccines- Covaxin and Covishield to other countries.
While Covishield is developed and patented by Oxford University and AstraZeneca and locally manufactured by Serum Institute of India (SII), Covaxin is an indigenous vaccine developed by Bharat Biotech but is not patented yet. India labelled this aid as ‘vaccine diplomacy’ and set an example of humanitarian aid in times of need.
However, there is now a problem- the lack of India’s foresight in securing the rights of its powerhouse research units by patenting Covaxin. India has pushed for the waiver of patents on vaccines which poses an issue regarding international trade and how the world deals with pharmaceutical products.
PATENTS AND INTERNATIONAL TRADE
Intellectual Property rights (IPR) aims to preserve the creators’ ownership over their intellect, which can be in the form of an invention, business name, a suit of software or a manuscript. Medical drugs fall under the category of ‘invention’, which is protected by patents. Engineering vaccines is a very complex medical process due to the requirement of technical “know-how and significant investment in research and development.” The patent holder of a vaccine is called the “Patentee”, who can prevent others from making, using, importing, or selling their vaccine for the patent’s life, which is approximately 20 years.
‘Process’ and ‘product’ are categories wherein a patent for a vaccine can be granted under the country’s domestic laws. There are certain treaties that regulate cross border patent regulation. Anyone interested in using their patent in a different country from that of the country of origin, can do so by applying for a patent under the legislation of the proposed country. Trade and access to patented medical products worldwide are regulated by the WTO Agreement on Trade-Related Aspects of Intellectual Property (TRIPS). All the contracting states of WTO should ensure that patents must be available for all technology fields, including medical technology like vaccines. Doha Declaration was issued in 2001 to synchronize the monopoly rights created in favour of the Patentee under TRIPS agreement with unreasonable restrain on international trade, especially concerning access to affordable medical products. The purpose of the declaration is to recognize the need to protect the IPR of evolving medicines while simultaneously addressing IPR on excessive prices and access to affordable health care.
INDIA’S STAND ON PATENTING VACCINES
India introduced a joint initiative with South Africa to waiver patent and other IPR on Covid-19 vaccines and drugs. In its document, India recognized how many countries are facing national emergencies and acute shortages of medical supplies and stressed the need for WTO members to ensure that “intellectual property rights such as patents, industrial designs, copyright and protection of undisclosed information doesn’t create barriers to the timely access to affordable medical products including vaccines and medicines or to scaling-up of research, development, manufacturing and supply of medical products essential to combat COVID-19.”
There were concerns expressed regarding how vaccines could be made available promptly and how IPR could hinder the timely distribution of affordable medical supplies to patients worldwide. It was also argued that many countries might face institutional and legal difficulties when using flexibilities available in the TRIPS Agreement. India and South Africa requested that the Council for TRIPS recommend a waiver to the General Council that would continue until most of the world’s population developed immunity through widespread vaccination.
After it became apparent that other countries sought to block the initiative, India urged WTO to consider, citing its own initiative to supply over 36 million vaccine doses to 35 countries. It was argued that if waiver didn’t result in an increase in manufacturing capacity, the commercial interests of existing IP holders would not be impacted. It was stressed that India and South Africa weren’t against IPR, but their sole motivation was the health emergency and, to a certain extent, the commercial interests of other companies in the country, like aviation and tourism.
NEED FOR GRANTING PATENTS
At present, India’s advocacy for IPR waiver on vaccines is contrary to the flow of development in the international trade law regime. It is necessary to understand the intention behind the need to maintain IPR protection despite its contrary nature in promoting fair international trade.
Patent incentivization for medical products, especially vaccines, strives for innovation in the field; otherwise, the scope of no returns on a heavy investment of vaccine production will hamper rapid growth in the invention of new medical products. Patent protection promotes the idea of development in future research of products by protecting investments in research and development. Waiver of patent rights is an immediate solution, but it is crucial to understand the long term goals of patent protection as undermining these rights would extinguish the rewards that inspire innovation, thus hampering the discovery of knowledge for novel products.
RECOMMENDATIONS FOR INDIA’S FUTURE POLICY
Various complexities are revolving around the process and duration of patent approval. Any proposal under TRIPS will also deal with several procedural requirements. Such issues might pose a hindrance against the recommendation of balance and ultimately favour waiver of patent rights. However, the ongoing pandemic is a novel challenge globally. The authors believe that after the developments of the Doha Declaration, there are various ways in which TRIPS provides novel solutions for the issue, which directs the focus away from the singular path of complete waiver.
The institution of TRIPS is modelled in a way that can resolve the “inherent tension between the protection of intellectual property and the need to make and distribute affordable medicines”. There are several recourses available that India can adopt, which are also available under the domestic laws of the country that promote fair trade and access to medical care while simultaneously protecting Patentee rights which are as followed: –
- Compulsory Licensing
Compulsory licensing is a method where a third party is allowed to produce a patent technology without the permission of the Patentee. Article 31 of the TRIPS agreement provides legal requirements to be followed for issuing a compulsory license. In a national public health emergency, compulsory licenses can be issued in a non-exclusive manner for a limited purpose. The utility would be limited to supply to the domestic market, and the patent holder must be paid ‘adequate renumeration’ for the compulsory license. Article 31bis of the TRIPS Agreement also provides a mechanism of importing patent products outside the domestic market to an underdeveloped country by issuing a Compulsory license.
In India, section 92 of the Indian Patent Act provides for a mechanism regarding the grant of a compulsory license in case if there is either a “national emergency” or “extreme urgency” or in cases of “public non-commercial use”. In 2012, India granted the first ever compulsory license to Hyderabad-based Natco Pharma to produce a generic version of Bayer’s Nexavar, an anti-cancer agent used in the treatment of liver kidney cancer.
Several countries have liberalized their legal requirements since the COVID-19 broke to fasten the process. Canada has passed legislation promoting rapid approval and grant of Compulsory licenses. Furthermore, Germany has also enacted the Prevention and Control of Infectious Diseases in Humans Act which allows the federal government to issue Compulsory licenses in case of a national epidemic and COVID-19 qualifies as one.
Therefore, to effectively ensure a balance between the patent holder’s rights and promote fair trade and access to health care, India can modify its position to make the existing national provision of the country more liberal on compulsory licensing and remove obstacles to their use.
- Voluntary Licensing
Voluntary licensing refers to IP-holders voluntarily granting licenses to their patents, which help the license holder produce and market the medicines at affordable cost in low or middle-income countries.
Voluntary Licenses make products more accessible as there are multiple companies that now manufacture the products, reducing the burden on the patent holder to manufacture and supply to distant places, where their local manufacturers could reach if they hold the license. It also helps in curbing monopolistic trade practices as one license could be issued to many generic companies.
An example of voluntary licenses in current times is of the licensing agreement between AstraZeneca and SII, where the latter has the license to produce the ‘Covishield’ vaccine doses for India and other developing countries, a move that WTO chief Dr. Ngozi Okonjo-Iweala praised.
- COVAX Facility
World Health Organization developed the ‘COVID-19 Vaccine Global Access Facility (COVAX) to promote global and equitable access to COVID-19 technologies, especially medical care. COVAX is a bilateral vaccine procurement mechanism wherein, depending on the fair allotment mechanism, vaccines shall be distributed amongst the member countries of WHO to vaccinate around 20 % of their population.
India’s inclusion in the COVAX facility provides a mechanism for the country to formulate an agreement with the facility to supply vaccine to developing countries, thereby providing an alternative to the existing proposal of waiver of patent rights.
- Technology Transfer Agreements
Article 66.2 of TRIPS created a legal obligation that reads: “Developed country members shall provide incentives to enterprises and institutions in their territories for the purpose of promoting and encouraging technology transfer to least-developed country members to enable them to create a sound and viable technological base.”
While this article doesn’t bind India due to its economic status, it could utilize its unique position by continuing its humanitarian efforts, but rather than distributing free vaccines, it could invest in the medical infrastructure of these countries by building hospitals, research centers and supplying laboratory equipment through aid, loans or by holding a per cent of the administrative and financial stakes of this infrastructure. While this initiative may take more time, financial and political efforts, the result would be long-lasting and concrete, simultaneously fostering a better bilateral relationship between India and the recipient country.
CONCLUSION
As India continues to push for waiver of patents of other vaccines, citing trade and humanitarian issues, it forgets something crucial- this benevolence comes at the cost of its research scientists. Failure to secure the IPR of the companies who developed the vaccines doesn’t just set a bad example; it pushes back the company’s ability to invest in further research, especially when the resources are limited and there are many beneficiaries.
As a global player, India should focus on empowering other nations so that they can manufacture the vaccines themselves. Moreover, patents will not necessarily hinder India’s efforts of securing vaccines for poorer countries, as has been made evident through the aforementioned recommendations.
The authors feel that the need of the hour is to explore the untapped potential of these countries in becoming self-sufficient, to prepare them for future calamities. A symbiotic relationship like this would not just help India’s foreign relations, but help make a better world altogether.
References
- G. Raizada & S.S. Dhillon, “Impact of Intellectual Property rights on International Trade: Evidence from India”, 22 JIPR 200 (2017).
- Milstien, J and Widdus, R “Facilitating Access to Vaccines: An Overview of Legal and Political Issues”, 1 (2) Pharm Dev Regul 101-116 (2003).
- WHO, “WTO and TRIPS Agreement”, available at: https://www.who.int/medicines/areas/policy/wto_trips/en/#:~:text=Patent%20protection%20The%20TRIPS%20Agreement,significantly%20shorter%20in%20many%20countries.
- The Patents Act, 1970.
- R. Elliott & M.H Bonin, “Patents, International Trade Law and Access to Essential Medicines”, CAEM 1-2 (2002)
- Article 27 TRIPS Agreement, 1995, available at: https://www.wto.org/english/docs_e/legal_e/27-trips_04c_e.htm
- WHO, The DOHA declaration on the trips agreement and public health, available at: https://www.who.int/medicines/areas/policy/doha_declaration/en/.
- Communication from India and South Africa, Waiver From Certain Provisions of the TRIPS Agreement for the Prevention, Containment and Treatment of COVID-19, Council for TRIPS, WTO, IP/C/W/669.
- WHO, “Sharing of influenza viruses and access to vaccines and other benefits: Interdisciplinary Working Group on Pandemic Influenza Preparedness”, INTERGOVERNMENTAL MEETING ON PANDEMIC INFLUENZA PREPAREDNESS: SHARING OF INFLUENZA VIRUSES AND ACCESS TO VACCINES AND OTHER BENEFITS, A/PIP/IGM/4 (2007).
- William D. Nordhaus, “Invention, Growth And Welfare: A Theoretical Treatment Of Technological Change”, 88-89 (1969).
- Jennifer Hillman, “Drugs and Vaccines Are Coming—But to Whom?,” Foreign Affairs, May 19, 2020.
- TRIPS Agreement, supra note 6.
- Id., Article 31 bis TRIPS Agreement, 1995.
- Section 92, The Patents Act, 1970
- R. Sivaraman, “Bayer Plea Against NATCO on cancer drug dismissed”, The Hindu, Sept. 16, 2012.
- Silverman E, A Canadian bill would make it easier to issue compulsory licenses for Covid-19 products, Available: https://www.statnews.com/pharmalot/2020/03/25/canada-compulsory-license-coronavirus-covid19/
- Houldsworth A, The key covid-19 compulsory licensing developments so far, 2020, Available: https://www.iam-media.com/coronavirus/the-key-covid-19-compulsory-licensing-developments-so-far.
- WHO, WHO Director-General’s opening remarks at the media briefing on COVID-19, 05 March 2021, available at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-5-march-2021.
- WHO, Fair allocation mechanism for COVID-19 vaccines through the COVAX Facility, Sept. 9, 2020, Available at: https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility
- Id.
















































































 onducted a total of five underground nuclear tests, breaking a 24-year self-imposed moratorium on nuclear testing. Pakistan followed, claiming 5 tests on May 28, 1998, and an additional test on May 30. The unannounced tests created a global storm of criticism, as well as a serious setback for decades of U.S. nuclear nonproliferation efforts in South Asia. On May 13, 1998, President Clinton imposed economic and military sanctions on India, mandated by Section 102 of the Arms Export Control Act (AECA), and applied the same sanctions to Pakistan on May 30. Some effects of the sanctions on India included: termination of $21 million in FY1998 economic development assistance; postponement of $1.7 billion in lending by the International Financial Institutions (IFI), as supported by the Group of Eight (G-8) leading industrial nations; prohibition on loans or credit from U.S. banks to the government of India; and termination of Foreign Military Sales under the Arms Export Control Act. Humanitarian assistance, food, or other agricultural commodities are excepted from sanctions under the law.
onducted a total of five underground nuclear tests, breaking a 24-year self-imposed moratorium on nuclear testing. Pakistan followed, claiming 5 tests on May 28, 1998, and an additional test on May 30. The unannounced tests created a global storm of criticism, as well as a serious setback for decades of U.S. nuclear nonproliferation efforts in South Asia. On May 13, 1998, President Clinton imposed economic and military sanctions on India, mandated by Section 102 of the Arms Export Control Act (AECA), and applied the same sanctions to Pakistan on May 30. Some effects of the sanctions on India included: termination of $21 million in FY1998 economic development assistance; postponement of $1.7 billion in lending by the International Financial Institutions (IFI), as supported by the Group of Eight (G-8) leading industrial nations; prohibition on loans or credit from U.S. banks to the government of India; and termination of Foreign Military Sales under the Arms Export Control Act. Humanitarian assistance, food, or other agricultural commodities are excepted from sanctions under the law. 
















 The first ministerial level meeting of QUAD was held on the sidelines of the United Nations General Assembly in New York. Before this, the QUAD had
The first ministerial level meeting of QUAD was held on the sidelines of the United Nations General Assembly in New York. Before this, the QUAD had AusIndEx is an exercise between India and Australia which was first held in 2015.The Australian
AusIndEx is an exercise between India and Australia which was first held in 2015.The Australian 







 On recommendations of the Japanese government, the four countries met at Manila, Philippines for ASEAN Regional Forum (ARF) originally, but also ended up having a meeting of what we call the first meeting of four nation states on issues of
On recommendations of the Japanese government, the four countries met at Manila, Philippines for ASEAN Regional Forum (ARF) originally, but also ended up having a meeting of what we call the first meeting of four nation states on issues of  On his official visit to India, Japanese PM Mr. Shinzo Abe reinforced the ties of two nations, i.e., Japan and India with his famous speech about
On his official visit to India, Japanese PM Mr. Shinzo Abe reinforced the ties of two nations, i.e., Japan and India with his famous speech about  In 2007, Japanese President Shinzo Abe resigned from his post citing health reasons. This had a significant impact on QUAD as he was the architect & advocate of QUAD. His successor, Yasuo Fukuda, did not take up QUAD with such zeal leading to dormancy of the forum. (
In 2007, Japanese President Shinzo Abe resigned from his post citing health reasons. This had a significant impact on QUAD as he was the architect & advocate of QUAD. His successor, Yasuo Fukuda, did not take up QUAD with such zeal leading to dormancy of the forum. (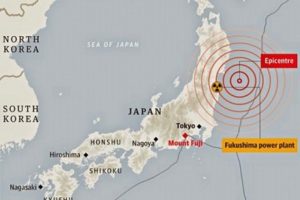 Japan earthquake and tsunami of 2011, also called Great Sendai Earthquake or Great Tōhoku Earthquake, was a 9.0 magnitude earthquake which struck below the floor of the Western Pacific at 2:49 PM. The powerful earthquake affected the northeastern coast of Honshu, Japan’s main island, and also initiated a series of large tsunami waves that devastated coastal areas of Japan, which also led to a major nuclear accident. Japan received aid from India, US, Australia as well as other countries. US Navy aircraft carrier was dispatched to the area and Australia sent search-and-rescue teams.
Japan earthquake and tsunami of 2011, also called Great Sendai Earthquake or Great Tōhoku Earthquake, was a 9.0 magnitude earthquake which struck below the floor of the Western Pacific at 2:49 PM. The powerful earthquake affected the northeastern coast of Honshu, Japan’s main island, and also initiated a series of large tsunami waves that devastated coastal areas of Japan, which also led to a major nuclear accident. Japan received aid from India, US, Australia as well as other countries. US Navy aircraft carrier was dispatched to the area and Australia sent search-and-rescue teams.  India and Australia signed the
India and Australia signed the  The India-Japan Agreement for Cooperation in the Peaceful Uses of Nuclear Energy was signed on 11 November, 2016 and came into force on 20 July, 2017 which was representative of strengthening ties between India and Japan. Diplomatic notes were exchanged between Dr. S. Jaishankar and H.E. Mr. Kenji Hiramatsu, Ambassador of Japan to India. (
The India-Japan Agreement for Cooperation in the Peaceful Uses of Nuclear Energy was signed on 11 November, 2016 and came into force on 20 July, 2017 which was representative of strengthening ties between India and Japan. Diplomatic notes were exchanged between Dr. S. Jaishankar and H.E. Mr. Kenji Hiramatsu, Ambassador of Japan to India. ( The foreign ministry
The foreign ministry The Officials of QUAD member countries met in Singapore on November 15, 2018 for consultation on regional & global issues of common interest. The main discussion revolved around connectivity, sustainable development, counter-terrorism, maritime and cyber security, with the view to promote peace, stability and prosperity in the
The Officials of QUAD member countries met in Singapore on November 15, 2018 for consultation on regional & global issues of common interest. The main discussion revolved around connectivity, sustainable development, counter-terrorism, maritime and cyber security, with the view to promote peace, stability and prosperity in the  The 23rd edition of trilateral Malabar maritime exercise between India, US and Japan took place on 26 September- 04 October, 2019 off the coast of Japan.
The 23rd edition of trilateral Malabar maritime exercise between India, US and Japan took place on 26 September- 04 October, 2019 off the coast of Japan.  After the first ministerial level meeting of QUAD in September, 2019, the senior officials of US, Japan, India and Australia again met for consultations in Bangkok on the margins of the East Asia Summit. Statements were issued separately by the four countries. Indian Ministry of External Affairs said “In statements issued separately by the four countries, MEA said, “proceeding from the strategic guidance of their Ministers, who met in New York City on the sidelines of the UN General Assembly recently, the officials exchanged views on ongoing and additional practical cooperation in the areas of connectivity and infrastructure development, and security matters, including counterterrorism, cyber and maritime security, with a view to promoting peace, security, stability, prosperity in the Indo-Pacific region.”
After the first ministerial level meeting of QUAD in September, 2019, the senior officials of US, Japan, India and Australia again met for consultations in Bangkok on the margins of the East Asia Summit. Statements were issued separately by the four countries. Indian Ministry of External Affairs said “In statements issued separately by the four countries, MEA said, “proceeding from the strategic guidance of their Ministers, who met in New York City on the sidelines of the UN General Assembly recently, the officials exchanged views on ongoing and additional practical cooperation in the areas of connectivity and infrastructure development, and security matters, including counterterrorism, cyber and maritime security, with a view to promoting peace, security, stability, prosperity in the Indo-Pacific region.” US 2+2 Ministerial Dialogue was held on 18 December, 2019, in Washington DC. Secretary of State Michael R. Pompeo and Secretary of Defense Mark T. Esper will host Indian Minister of External Affairs Dr. S. Jaishankar and Minister of Defense Shri Rajnath Singh. The discussion focussed on deepening bilateral strategic and defense cooperation, exchanging perspectives on global developments, and our shared leadership in the Indo-Pacific region.The two democracies signed the Industrial Security Annex before the 2+2 Dialogue. Assessments of the situation in Afghanistan, Pakistan, Nepal, Sri Lanka, and the Indian Ocean region in general were shared between both countries. (
US 2+2 Ministerial Dialogue was held on 18 December, 2019, in Washington DC. Secretary of State Michael R. Pompeo and Secretary of Defense Mark T. Esper will host Indian Minister of External Affairs Dr. S. Jaishankar and Minister of Defense Shri Rajnath Singh. The discussion focussed on deepening bilateral strategic and defense cooperation, exchanging perspectives on global developments, and our shared leadership in the Indo-Pacific region.The two democracies signed the Industrial Security Annex before the 2+2 Dialogue. Assessments of the situation in Afghanistan, Pakistan, Nepal, Sri Lanka, and the Indian Ocean region in general were shared between both countries. ( The foreign ministers of QUAD continued their discussions from the last ministerial level meeting in 2019, on 6 October, 2020. While there was no joint statement released, all countries issued individual readouts. As per the issue readout by India, the discussion called for a coordinated response to the challenges including financial problems emanating from the pandemic, best practices to combat Covid-19, increasing the resilience of supply chains, and enhancing access to affordable vaccines, medicines and medical equipment. There was also a focus on maintaining stability in the Indo-Pacific region amidst growing tensions. Australian media release mentions “We emphasised that, especially during a pandemic, it was vital that states work to ease tensions and avoid exacerbating long-standing disputes, work to counter disinformation, and refrain from malicious cyberspace activity. Ministers reiterated that states cannot assert maritime claims that are inconsistent with international law, particularly the United Nations Convention on the Law of the Sea (UNCLOS).”
The foreign ministers of QUAD continued their discussions from the last ministerial level meeting in 2019, on 6 October, 2020. While there was no joint statement released, all countries issued individual readouts. As per the issue readout by India, the discussion called for a coordinated response to the challenges including financial problems emanating from the pandemic, best practices to combat Covid-19, increasing the resilience of supply chains, and enhancing access to affordable vaccines, medicines and medical equipment. There was also a focus on maintaining stability in the Indo-Pacific region amidst growing tensions. Australian media release mentions “We emphasised that, especially during a pandemic, it was vital that states work to ease tensions and avoid exacerbating long-standing disputes, work to counter disinformation, and refrain from malicious cyberspace activity. Ministers reiterated that states cannot assert maritime claims that are inconsistent with international law, particularly the United Nations Convention on the Law of the Sea (UNCLOS).” On September 24, President Biden hosted Prime Minister Scott Morrison of Australia, Prime Minister Narendra Modi of India, and Prime Minister Yoshihide Suga of Japan at the White House for the first-ever in-person Leaders’ Summit of the QUAD. The leaders released a Joint Statement which summarised their dialogue and future course of action. The regional security of the Indo-Pacific and strong confidence in the ASEAN remained on the focus along with response to the Pandemic.
On September 24, President Biden hosted Prime Minister Scott Morrison of Australia, Prime Minister Narendra Modi of India, and Prime Minister Yoshihide Suga of Japan at the White House for the first-ever in-person Leaders’ Summit of the QUAD. The leaders released a Joint Statement which summarised their dialogue and future course of action. The regional security of the Indo-Pacific and strong confidence in the ASEAN remained on the focus along with response to the Pandemic.  The QUAD Vaccine Partnership was announced at the first QUAD Summit on 12 March 2021 where QUAD countries agreed to deliver 1.2 billion vaccine doses globally. The aim was to expand and finance vaccine manufacturing and equipping the Indo-Pacific to build resilience against Covid-19. The launch of a senior-level QUAD Vaccine Experts Group, comprised of top scientists and officials from all QUAD member governments was also spearheaded.
The QUAD Vaccine Partnership was announced at the first QUAD Summit on 12 March 2021 where QUAD countries agreed to deliver 1.2 billion vaccine doses globally. The aim was to expand and finance vaccine manufacturing and equipping the Indo-Pacific to build resilience against Covid-19. The launch of a senior-level QUAD Vaccine Experts Group, comprised of top scientists and officials from all QUAD member governments was also spearheaded.  Although the Tsunami Core group had to be disbanded on fulfilment of its purpose, however the quadrilateral template that formed remained intact as a successful scaffolding of four countries, as stated by authors Patrick Gerard Buchan and Benjamin Rimland in their diplomatic brief about QUAD ( you can access the brief at
Although the Tsunami Core group had to be disbanded on fulfilment of its purpose, however the quadrilateral template that formed remained intact as a successful scaffolding of four countries, as stated by authors Patrick Gerard Buchan and Benjamin Rimland in their diplomatic brief about QUAD ( you can access the brief at  Secretary of State Colin Powell stated that the Core Tsunami Group was to be disbanded and folded and clubbed with the broader United Nations led Relief Operations. In a Tsunami Relief Conference in Jakarta, Secretary Powell stated that
Secretary of State Colin Powell stated that the Core Tsunami Group was to be disbanded and folded and clubbed with the broader United Nations led Relief Operations. In a Tsunami Relief Conference in Jakarta, Secretary Powell stated that  Soon after the Earthquake and Tsunami crisis, humanitarian reliefs by countries, viz., US, India, Japan, and Australia started to help the 13 havoc-stricken countries. The US initially promised $ 35 Millions in aid. However, on 29
Soon after the Earthquake and Tsunami crisis, humanitarian reliefs by countries, viz., US, India, Japan, and Australia started to help the 13 havoc-stricken countries. The US initially promised $ 35 Millions in aid. However, on 29 At 7:59AM local time, an earthquake of 9.1 magnitude (undersea) hit the coast of Sumatra, an Indonesian island. As a result of the same, massive waves of Tsunami triggered by the earthquake wreaked havoc for 7 hours across the Indian Ocean and to the coastal areas as far away as East Africa. The infamous Tsunami killed around 225,000 people, with people reporting the height of waves to be as high as 9 metres, i.e., 30 feet. Indonesia, Srilanka, India, Maldives, Thailand sustained horrendously massive damage, with the death toll exceeding 200,000 in Northern Sumatra’s Ache province alone. A great many people, i.e., around tens of thousands were found dead or missing in Srilanka and India, mostly from Andaman and Nicobar Islands of Indian territory. Maldives, being a low-lying country, also reported casualties in hundreds and more, with several non-Asian tourists reported dead or missing who were vacationing. Lack of food, water, medicines burgeoned the numbers of casualties, with the relief workers finding it difficult to reach the remotest areas where roads were destroyed or civil war raged. Long-term environmental damage ensued too, as both natural and man-made resources got demolished and diminished.
At 7:59AM local time, an earthquake of 9.1 magnitude (undersea) hit the coast of Sumatra, an Indonesian island. As a result of the same, massive waves of Tsunami triggered by the earthquake wreaked havoc for 7 hours across the Indian Ocean and to the coastal areas as far away as East Africa. The infamous Tsunami killed around 225,000 people, with people reporting the height of waves to be as high as 9 metres, i.e., 30 feet. Indonesia, Srilanka, India, Maldives, Thailand sustained horrendously massive damage, with the death toll exceeding 200,000 in Northern Sumatra’s Ache province alone. A great many people, i.e., around tens of thousands were found dead or missing in Srilanka and India, mostly from Andaman and Nicobar Islands of Indian territory. Maldives, being a low-lying country, also reported casualties in hundreds and more, with several non-Asian tourists reported dead or missing who were vacationing. Lack of food, water, medicines burgeoned the numbers of casualties, with the relief workers finding it difficult to reach the remotest areas where roads were destroyed or civil war raged. Long-term environmental damage ensued too, as both natural and man-made resources got demolished and diminished.
No responses yet