
– By Ashish Gosain
– The Author is an Intellectual Property and Legal practitioner and a PhD scholar in Science Policy at Jawaharlal Nehru University, New-Delhi.
Ever since the impetus on economic globalization began, a key aspect of the Trade and Technology debate has been the creation of capabilities in domestic Industry, to enable them to compete in the International markets, while responding to the needs of their domestic constituency and society. This is markedly the case in the Pharmaceuticals Industry. The need of enabling technology-oriented growth requires the critical mass of Learning opportunities. This has to be aligned with the redistributive goals of access to medicines in a developing country context.
As the global integration of economies continues and increases in foreign FDI flows is touted to be the next big thing, there emerges tensions between the processes of internationalization on the one hand and maximizing welfare in society on the other. The primary burden on domestic Institutions in this context, thus need not be over-emphasised. It is here the role of multilateral institutions has come under sharp focus in export-oriented economies like that of India. In the post TRIPS era, countries are grappling with the failed promise of multilateral institutions, in terms of redistributive justice. Today, as the World confronts the worst pandemic in a hundred years, the situation has brought to centre stage, certain priorities that seek to re-emphasize the notion of regulatory sovereignty.
The particular context is that of the policy space that countries have in relation to recalibrating the structural nature of developing economies to enhance developmental outcomes from Trade in Technology. As the pressures on Domestic healthcare systems brings them to brink of collapse, the need for supportive institutions that heal the world onto path out of this pandemic are crucial. In a previous conceptualization, Healthcare in resource constrained settings was forced to focus on provisioning of Infrastructure under a Public Goods paradigm. This is more so today, in view of Healthcare 4.0, where the focus shifts to generation of patient outcomes. One such institution is the promotion of Innovation that brings the competing mandates of social welfare provisioning and providing long-term incentives for innovation. Strengthened Intellectual Property protection in terms of TRIPS plus standards is having a negative impact on affordability and accessibility of medicines. Thus, calls for serious and collective efforts at reform is the need of the Hour. This increasing property rights protection, coupled with the lack of R & D productivity by vertically integrated firms (Ippoliti et al, 2019) for treatment in areas of disease burden, coupled with an inability to learn from past experience are making the current situation more and more difficult. The need to adopt a humanitarian approach to the pandemic and the challenges that it poses will serve to reinstate some of the faith that Nations have in the ability of international Institutions to moderate trade and economic imperatives with needs of society.
The flexibilities in the TRIPS framework (Trade related Intellectual Property Rights) have retained welfarist objectives after a hard-fought battle by civil society, activists, international organisations etc. The developments that lead to the Doha Ministerial Declaration had breathed life into the prospect of access to medicines. However, in the successive decades since, the momentum on access to medicines has suffered numerous setbacks. While the problems associated with proprietary regimes with public health emergencies has been a consistent theme, the continuing review of it’s alignment with developmental priorities is in question. The trend of hedonistic regionalism that has been spawned in the last decade is a retreat from the initial promise of globalization. Today, with the trends of “Vaccine Nationalism” being seen, which is in violation of jus cogens, a cardinal principle of International Law, Nations scurry to stockpile reserves, impose newer restrictions on international migration and impose trade curbs on exports, through emergency measures justified under Domestic Laws. This coupled with the regulatory capitalism imposed on countries, in the name of incentives of innovation has to take a view at the Internalisation dimension in the Ownership Location and Internalisation Paradigm. If there is anything in the name of international solidarity, in humanity’s darkest hour, Developed countries should part with control over resources needed to enable the World to overcome this crisis.
For this to happen, the leadership must come from multilateral Institutions, which have hitherto faced a democratic deficit, in regard to their assumption of an agenda setting role. The disappointment over the functioning of the World Health Organization in facilitating soft Law approaches towards domestic Disease surveillance has come under severe criticism, since the outbreak of the CoVID-19 virus. This is on account of the underlying institutions that enable trade in technology in this Sector. Secondly, while there has been a mobilization of actors of various types in enabling domestic Innovation systems, yet the regulatory spillovers sustaining this process of technology led growth must not loose sight of the structural features of the economic structure of countries , coupled with their developmental status. A key question arises in relation to the structural resilience of Innovation value chains supporting Healthcare as a Sustainable Development Goal. The transition period given to Less developed countries should be enhanced so as to facilitate domestic capacity building under non-proprietary regimes of Intellectual Property. For other transition economies, actuating the critical mass of learning opportunities from global participation must be done (Pietrobelli and Rabelloti, 2009). All in all, the real promise of globalization lies in generating the structures that facilitate and promote greater cohesion and international collaboration among a diversity of actors to promote healthcare provisioning in the World on a long-term basis.
References
- Foreign Direct Investment inflows into Pharmaceuticals sector in the period April 2020- December, 2020 was 9234 crore rupees (USD 1246 Million); In April 2019 – March 2020 was 3650 crore rupees (USD 518 Million) and 1842 crore rupees (USD 266 Million) in FY 2018-19. (Source: Minister of State for Shipping and Chemicals and Fertiliser’s Reply to Parliament). For recent policy movements see Kirtika Suneja, “Mega FDI plan to focus on faster pharma approvals”, Economic Times (10th May, 2020)Online at: https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/mega-fdi-plan-to-focus-on-faster-pharma-approvals/articleshow/75664593.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst.
- Declaration on Patent Protection-Regulatory Sovereignty under TRIPS agreement, available online at : https://www.mpg.de/8133454/Patent-Declaration1.pdf . The author participated in a workshop on the Deliberations of the Declaration held in New Delhi during 2017.
- Mohammed El Said, “Radical Approaches during Unusual Circumstances: Intellectual Property regulation and the CoVID -19 Dilemma,” Development, DOI: 10.1057/s41301-020-00257-x
- Roberto Ippoliti et al, “Partnership and Innovation in the Pharmaceutical Industry: the case of clinical research”, Economics of Innovation and New Technology”, DOI: 10.1080/10438599.2019.1701782.
- The Year 2020 marked the 18th Anniversary of the Doha Ministerial Declaration on Trips agreement and Public Health.
- “Foreign Direct Investment: The OLI framework”, University of Oxford, available online at : http://users.ox.ac.uk/~econ0211/papers/pdf/fdiprinceton.pdf . One of it’s key proponents, John Dunning viewed knowledge as “Ownership advantages”.
- Carlo Pietrobelli et al (2007), “ Global Value Chains and Technological Capabilities: A Framework to Study Industrial Innovation in Developing Countries” , DOI: 10.4324/9780203937396.ch6 .
















































































 onducted a total of five underground nuclear tests, breaking a 24-year self-imposed moratorium on nuclear testing. Pakistan followed, claiming 5 tests on May 28, 1998, and an additional test on May 30. The unannounced tests created a global storm of criticism, as well as a serious setback for decades of U.S. nuclear nonproliferation efforts in South Asia. On May 13, 1998, President Clinton imposed economic and military sanctions on India, mandated by Section 102 of the Arms Export Control Act (AECA), and applied the same sanctions to Pakistan on May 30. Some effects of the sanctions on India included: termination of $21 million in FY1998 economic development assistance; postponement of $1.7 billion in lending by the International Financial Institutions (IFI), as supported by the Group of Eight (G-8) leading industrial nations; prohibition on loans or credit from U.S. banks to the government of India; and termination of Foreign Military Sales under the Arms Export Control Act. Humanitarian assistance, food, or other agricultural commodities are excepted from sanctions under the law.
onducted a total of five underground nuclear tests, breaking a 24-year self-imposed moratorium on nuclear testing. Pakistan followed, claiming 5 tests on May 28, 1998, and an additional test on May 30. The unannounced tests created a global storm of criticism, as well as a serious setback for decades of U.S. nuclear nonproliferation efforts in South Asia. On May 13, 1998, President Clinton imposed economic and military sanctions on India, mandated by Section 102 of the Arms Export Control Act (AECA), and applied the same sanctions to Pakistan on May 30. Some effects of the sanctions on India included: termination of $21 million in FY1998 economic development assistance; postponement of $1.7 billion in lending by the International Financial Institutions (IFI), as supported by the Group of Eight (G-8) leading industrial nations; prohibition on loans or credit from U.S. banks to the government of India; and termination of Foreign Military Sales under the Arms Export Control Act. Humanitarian assistance, food, or other agricultural commodities are excepted from sanctions under the law. 
















 The first ministerial level meeting of QUAD was held on the sidelines of the United Nations General Assembly in New York. Before this, the QUAD had
The first ministerial level meeting of QUAD was held on the sidelines of the United Nations General Assembly in New York. Before this, the QUAD had AusIndEx is an exercise between India and Australia which was first held in 2015.The Australian
AusIndEx is an exercise between India and Australia which was first held in 2015.The Australian 







 On recommendations of the Japanese government, the four countries met at Manila, Philippines for ASEAN Regional Forum (ARF) originally, but also ended up having a meeting of what we call the first meeting of four nation states on issues of
On recommendations of the Japanese government, the four countries met at Manila, Philippines for ASEAN Regional Forum (ARF) originally, but also ended up having a meeting of what we call the first meeting of four nation states on issues of  On his official visit to India, Japanese PM Mr. Shinzo Abe reinforced the ties of two nations, i.e., Japan and India with his famous speech about
On his official visit to India, Japanese PM Mr. Shinzo Abe reinforced the ties of two nations, i.e., Japan and India with his famous speech about  In 2007, Japanese President Shinzo Abe resigned from his post citing health reasons. This had a significant impact on QUAD as he was the architect & advocate of QUAD. His successor, Yasuo Fukuda, did not take up QUAD with such zeal leading to dormancy of the forum. (
In 2007, Japanese President Shinzo Abe resigned from his post citing health reasons. This had a significant impact on QUAD as he was the architect & advocate of QUAD. His successor, Yasuo Fukuda, did not take up QUAD with such zeal leading to dormancy of the forum. (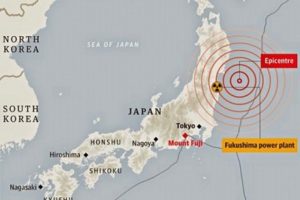 Japan earthquake and tsunami of 2011, also called Great Sendai Earthquake or Great Tōhoku Earthquake, was a 9.0 magnitude earthquake which struck below the floor of the Western Pacific at 2:49 PM. The powerful earthquake affected the northeastern coast of Honshu, Japan’s main island, and also initiated a series of large tsunami waves that devastated coastal areas of Japan, which also led to a major nuclear accident. Japan received aid from India, US, Australia as well as other countries. US Navy aircraft carrier was dispatched to the area and Australia sent search-and-rescue teams.
Japan earthquake and tsunami of 2011, also called Great Sendai Earthquake or Great Tōhoku Earthquake, was a 9.0 magnitude earthquake which struck below the floor of the Western Pacific at 2:49 PM. The powerful earthquake affected the northeastern coast of Honshu, Japan’s main island, and also initiated a series of large tsunami waves that devastated coastal areas of Japan, which also led to a major nuclear accident. Japan received aid from India, US, Australia as well as other countries. US Navy aircraft carrier was dispatched to the area and Australia sent search-and-rescue teams.  India and Australia signed the
India and Australia signed the  The India-Japan Agreement for Cooperation in the Peaceful Uses of Nuclear Energy was signed on 11 November, 2016 and came into force on 20 July, 2017 which was representative of strengthening ties between India and Japan. Diplomatic notes were exchanged between Dr. S. Jaishankar and H.E. Mr. Kenji Hiramatsu, Ambassador of Japan to India. (
The India-Japan Agreement for Cooperation in the Peaceful Uses of Nuclear Energy was signed on 11 November, 2016 and came into force on 20 July, 2017 which was representative of strengthening ties between India and Japan. Diplomatic notes were exchanged between Dr. S. Jaishankar and H.E. Mr. Kenji Hiramatsu, Ambassador of Japan to India. ( The foreign ministry
The foreign ministry The Officials of QUAD member countries met in Singapore on November 15, 2018 for consultation on regional & global issues of common interest. The main discussion revolved around connectivity, sustainable development, counter-terrorism, maritime and cyber security, with the view to promote peace, stability and prosperity in the
The Officials of QUAD member countries met in Singapore on November 15, 2018 for consultation on regional & global issues of common interest. The main discussion revolved around connectivity, sustainable development, counter-terrorism, maritime and cyber security, with the view to promote peace, stability and prosperity in the  The 23rd edition of trilateral Malabar maritime exercise between India, US and Japan took place on 26 September- 04 October, 2019 off the coast of Japan.
The 23rd edition of trilateral Malabar maritime exercise between India, US and Japan took place on 26 September- 04 October, 2019 off the coast of Japan.  After the first ministerial level meeting of QUAD in September, 2019, the senior officials of US, Japan, India and Australia again met for consultations in Bangkok on the margins of the East Asia Summit. Statements were issued separately by the four countries. Indian Ministry of External Affairs said “In statements issued separately by the four countries, MEA said, “proceeding from the strategic guidance of their Ministers, who met in New York City on the sidelines of the UN General Assembly recently, the officials exchanged views on ongoing and additional practical cooperation in the areas of connectivity and infrastructure development, and security matters, including counterterrorism, cyber and maritime security, with a view to promoting peace, security, stability, prosperity in the Indo-Pacific region.”
After the first ministerial level meeting of QUAD in September, 2019, the senior officials of US, Japan, India and Australia again met for consultations in Bangkok on the margins of the East Asia Summit. Statements were issued separately by the four countries. Indian Ministry of External Affairs said “In statements issued separately by the four countries, MEA said, “proceeding from the strategic guidance of their Ministers, who met in New York City on the sidelines of the UN General Assembly recently, the officials exchanged views on ongoing and additional practical cooperation in the areas of connectivity and infrastructure development, and security matters, including counterterrorism, cyber and maritime security, with a view to promoting peace, security, stability, prosperity in the Indo-Pacific region.” US 2+2 Ministerial Dialogue was held on 18 December, 2019, in Washington DC. Secretary of State Michael R. Pompeo and Secretary of Defense Mark T. Esper will host Indian Minister of External Affairs Dr. S. Jaishankar and Minister of Defense Shri Rajnath Singh. The discussion focussed on deepening bilateral strategic and defense cooperation, exchanging perspectives on global developments, and our shared leadership in the Indo-Pacific region.The two democracies signed the Industrial Security Annex before the 2+2 Dialogue. Assessments of the situation in Afghanistan, Pakistan, Nepal, Sri Lanka, and the Indian Ocean region in general were shared between both countries. (
US 2+2 Ministerial Dialogue was held on 18 December, 2019, in Washington DC. Secretary of State Michael R. Pompeo and Secretary of Defense Mark T. Esper will host Indian Minister of External Affairs Dr. S. Jaishankar and Minister of Defense Shri Rajnath Singh. The discussion focussed on deepening bilateral strategic and defense cooperation, exchanging perspectives on global developments, and our shared leadership in the Indo-Pacific region.The two democracies signed the Industrial Security Annex before the 2+2 Dialogue. Assessments of the situation in Afghanistan, Pakistan, Nepal, Sri Lanka, and the Indian Ocean region in general were shared between both countries. ( The foreign ministers of QUAD continued their discussions from the last ministerial level meeting in 2019, on 6 October, 2020. While there was no joint statement released, all countries issued individual readouts. As per the issue readout by India, the discussion called for a coordinated response to the challenges including financial problems emanating from the pandemic, best practices to combat Covid-19, increasing the resilience of supply chains, and enhancing access to affordable vaccines, medicines and medical equipment. There was also a focus on maintaining stability in the Indo-Pacific region amidst growing tensions. Australian media release mentions “We emphasised that, especially during a pandemic, it was vital that states work to ease tensions and avoid exacerbating long-standing disputes, work to counter disinformation, and refrain from malicious cyberspace activity. Ministers reiterated that states cannot assert maritime claims that are inconsistent with international law, particularly the United Nations Convention on the Law of the Sea (UNCLOS).”
The foreign ministers of QUAD continued their discussions from the last ministerial level meeting in 2019, on 6 October, 2020. While there was no joint statement released, all countries issued individual readouts. As per the issue readout by India, the discussion called for a coordinated response to the challenges including financial problems emanating from the pandemic, best practices to combat Covid-19, increasing the resilience of supply chains, and enhancing access to affordable vaccines, medicines and medical equipment. There was also a focus on maintaining stability in the Indo-Pacific region amidst growing tensions. Australian media release mentions “We emphasised that, especially during a pandemic, it was vital that states work to ease tensions and avoid exacerbating long-standing disputes, work to counter disinformation, and refrain from malicious cyberspace activity. Ministers reiterated that states cannot assert maritime claims that are inconsistent with international law, particularly the United Nations Convention on the Law of the Sea (UNCLOS).” On September 24, President Biden hosted Prime Minister Scott Morrison of Australia, Prime Minister Narendra Modi of India, and Prime Minister Yoshihide Suga of Japan at the White House for the first-ever in-person Leaders’ Summit of the QUAD. The leaders released a Joint Statement which summarised their dialogue and future course of action. The regional security of the Indo-Pacific and strong confidence in the ASEAN remained on the focus along with response to the Pandemic.
On September 24, President Biden hosted Prime Minister Scott Morrison of Australia, Prime Minister Narendra Modi of India, and Prime Minister Yoshihide Suga of Japan at the White House for the first-ever in-person Leaders’ Summit of the QUAD. The leaders released a Joint Statement which summarised their dialogue and future course of action. The regional security of the Indo-Pacific and strong confidence in the ASEAN remained on the focus along with response to the Pandemic.  The QUAD Vaccine Partnership was announced at the first QUAD Summit on 12 March 2021 where QUAD countries agreed to deliver 1.2 billion vaccine doses globally. The aim was to expand and finance vaccine manufacturing and equipping the Indo-Pacific to build resilience against Covid-19. The launch of a senior-level QUAD Vaccine Experts Group, comprised of top scientists and officials from all QUAD member governments was also spearheaded.
The QUAD Vaccine Partnership was announced at the first QUAD Summit on 12 March 2021 where QUAD countries agreed to deliver 1.2 billion vaccine doses globally. The aim was to expand and finance vaccine manufacturing and equipping the Indo-Pacific to build resilience against Covid-19. The launch of a senior-level QUAD Vaccine Experts Group, comprised of top scientists and officials from all QUAD member governments was also spearheaded.  Although the Tsunami Core group had to be disbanded on fulfilment of its purpose, however the quadrilateral template that formed remained intact as a successful scaffolding of four countries, as stated by authors Patrick Gerard Buchan and Benjamin Rimland in their diplomatic brief about QUAD ( you can access the brief at
Although the Tsunami Core group had to be disbanded on fulfilment of its purpose, however the quadrilateral template that formed remained intact as a successful scaffolding of four countries, as stated by authors Patrick Gerard Buchan and Benjamin Rimland in their diplomatic brief about QUAD ( you can access the brief at  Secretary of State Colin Powell stated that the Core Tsunami Group was to be disbanded and folded and clubbed with the broader United Nations led Relief Operations. In a Tsunami Relief Conference in Jakarta, Secretary Powell stated that
Secretary of State Colin Powell stated that the Core Tsunami Group was to be disbanded and folded and clubbed with the broader United Nations led Relief Operations. In a Tsunami Relief Conference in Jakarta, Secretary Powell stated that  Soon after the Earthquake and Tsunami crisis, humanitarian reliefs by countries, viz., US, India, Japan, and Australia started to help the 13 havoc-stricken countries. The US initially promised $ 35 Millions in aid. However, on 29
Soon after the Earthquake and Tsunami crisis, humanitarian reliefs by countries, viz., US, India, Japan, and Australia started to help the 13 havoc-stricken countries. The US initially promised $ 35 Millions in aid. However, on 29 At 7:59AM local time, an earthquake of 9.1 magnitude (undersea) hit the coast of Sumatra, an Indonesian island. As a result of the same, massive waves of Tsunami triggered by the earthquake wreaked havoc for 7 hours across the Indian Ocean and to the coastal areas as far away as East Africa. The infamous Tsunami killed around 225,000 people, with people reporting the height of waves to be as high as 9 metres, i.e., 30 feet. Indonesia, Srilanka, India, Maldives, Thailand sustained horrendously massive damage, with the death toll exceeding 200,000 in Northern Sumatra’s Ache province alone. A great many people, i.e., around tens of thousands were found dead or missing in Srilanka and India, mostly from Andaman and Nicobar Islands of Indian territory. Maldives, being a low-lying country, also reported casualties in hundreds and more, with several non-Asian tourists reported dead or missing who were vacationing. Lack of food, water, medicines burgeoned the numbers of casualties, with the relief workers finding it difficult to reach the remotest areas where roads were destroyed or civil war raged. Long-term environmental damage ensued too, as both natural and man-made resources got demolished and diminished.
At 7:59AM local time, an earthquake of 9.1 magnitude (undersea) hit the coast of Sumatra, an Indonesian island. As a result of the same, massive waves of Tsunami triggered by the earthquake wreaked havoc for 7 hours across the Indian Ocean and to the coastal areas as far away as East Africa. The infamous Tsunami killed around 225,000 people, with people reporting the height of waves to be as high as 9 metres, i.e., 30 feet. Indonesia, Srilanka, India, Maldives, Thailand sustained horrendously massive damage, with the death toll exceeding 200,000 in Northern Sumatra’s Ache province alone. A great many people, i.e., around tens of thousands were found dead or missing in Srilanka and India, mostly from Andaman and Nicobar Islands of Indian territory. Maldives, being a low-lying country, also reported casualties in hundreds and more, with several non-Asian tourists reported dead or missing who were vacationing. Lack of food, water, medicines burgeoned the numbers of casualties, with the relief workers finding it difficult to reach the remotest areas where roads were destroyed or civil war raged. Long-term environmental damage ensued too, as both natural and man-made resources got demolished and diminished.
No responses yet